ABSTRACT
INTRODUCTION
BASIC MECHANISMS OF SLEEP AND SLEEP DYSREGULATION
MELATONIN
GABA
CORTISOL
BLOOD GLUCOSE DYSREGULATION
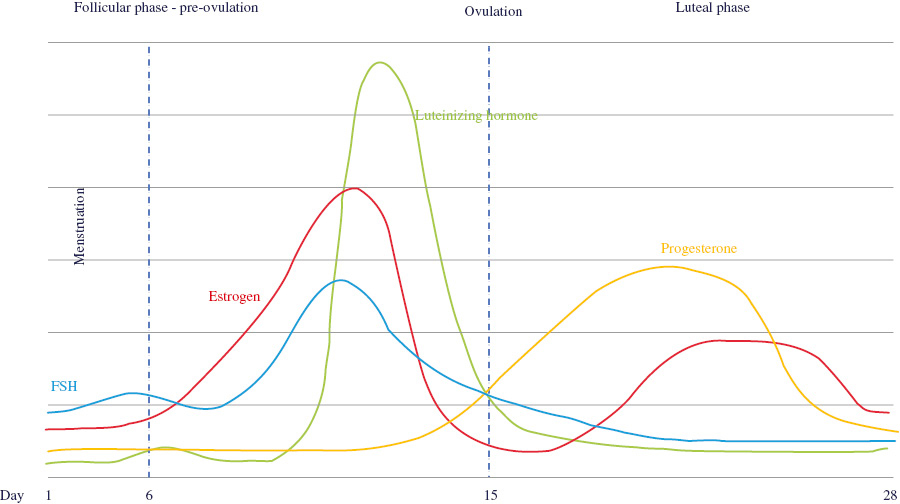
THE MENSTRUAL CYCLE: AN OVERVIEW
SLEEP AND THE MENSTRUAL CYCLE
SLEEP AND GLUCOSE METABOLISM
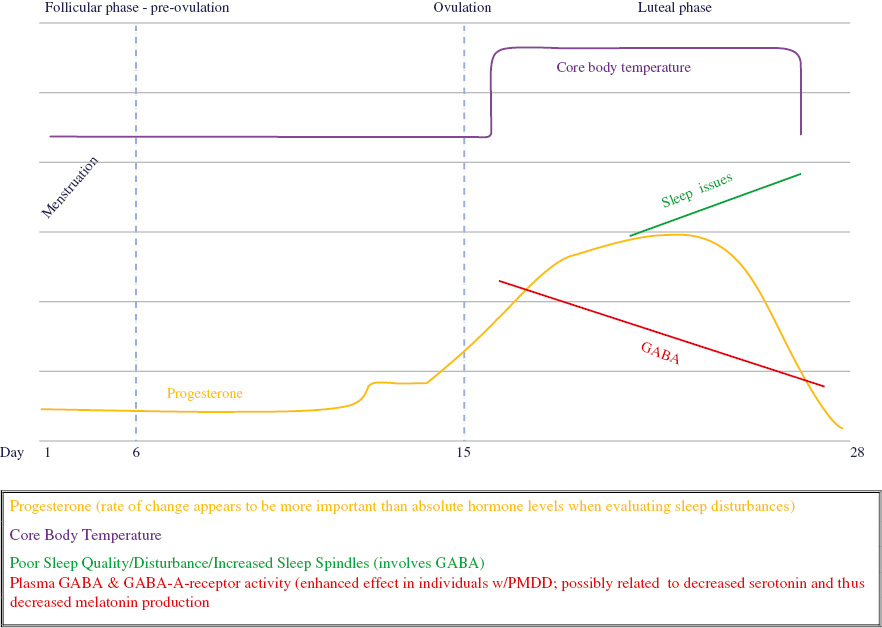
THE RELATIONSHIP OF PROGESTERONE, MELATONIN, GABA, AND CBT TO SLEEP DISTURBANCE: A THEORETICAL MODEL
CLINICAL IMPLICATIONS
-
Minimize need for pharmaceutical sleep-aids, which often have harmful side effects
-
Reduce the risk of certain chronic diseases (e.g. hypertension, T2DM, and obesity)
-
Improve mood, energy, and cognition
CONCLUSION
| Phase/event | Description | Hormonal changes (non-fertilized state) | |
|---|---|---|---|
| Follicular | • | Starts on first day of menstruation | FSH: starts low, ↑ ˜day 3, drops slightly, peaks ˜day 12, ↓LH: ↑ sharply ˜day 12, ↓ 16–32 hours before ovulationEstradiol: ↑ peaks ˜day 12 & then ↓Progesterone: starts ↑ around day 12 |
| • | Menstruation lasts between 3 and 7 days, 5 on average | ||
| • | Infertile during early phase | ||
| • | Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) are produced by the pituitary gland | ||
| • | LH and FSH promote ovulation and stimulate the ovaries to produce estrogen and progesterone | ||
| • | Ends with ovulation around day 16 or 17 | ||
| • | This phase varies in length | ||
| Ovulation | • | Egg release stimulated by increases in FSH and LH | |
| • | Lining of uterus continues to thicken | ||
| Luteal | • | Starts after ovulation | FSH: low |
| • | Lining of uterus thickens | LH: low | |
| • | Unless fertilization occurs, generally lasts 14 days | Estradiol: starts low, peaks between days 20 & 24 (lower peak than during follicular phase), ends low | |
| • | The ruptured follicle forms a corpus luteum, which produces progesterone | ||
| • | Body temperature increases slightly | Progesterone: increases to a peak around day 25 and then drops | |
| Neurotransmitter/hormone | Function | Menstrual cycle impacts |
| GABA | Inhibitory neurotransmitter that promotes sleep. GABAergic neurons are involved in the formation of sleep spindles. | Decreased during late luteal phase due to increased progesterone levels. Progesterone interacts with GABA-A receptors. Women with PMS may have reduced GABA-A receptor sensitivity. |
| Melatonin | Regulates sleep-wake cycles. | Affected by PMDD both in timing and amount of secretion. Can also be affected by stressors (via increased cortisol) during late luteal phase. Progesterone also competitively binds to corticosteroid binding globulin, potentially increasing available cortisol and further suppressing melatonin. |
| Serotonin | Maintains wakefulness and muscle tone. Not active during REM. Inhibited by GABAergic neurons. | According to Wihlbäck et al.74 there are phase-related differences in serotonin uptake sites and receptors. Decreased number of serotonin binding sites during progesterone’s peak during the luteal phase. |
| Glutamate | Precursor for GABA. | Serum levels were found to be inversely correlated with progesterone and estrogen levels.75 |
| Hypocretin (or orexin) | Stimulates release of glutamate, acts as an excitatory neurotransmitter, and is involved in regulation of sleep and arousal. | Literature search did not locate studies that investigated a direct sleep/menstrual cycle connection. |
| Acetylcholine | Involved in wakefulness and REM phase of sleep. | Literature search did not locate studies that investigated a direct sleep/menstrual cycle/acetylcholine connection. One small study of young women found that gonadal hormones did not appear to have an effect on plasma acetylcholine levels.76 According to Valera et al.77 progesterone “may interact with the acetylcholine binding site,” but does inhibit acetylcholine competitively. |
| Histamine | Wakefulness results from histamine or H1 receptor agonists while H1 antagonists have the opposite effect.78 Histamine-releasing neurons are not active during REM and NREM phases of sleep.78 GABAergic neurons turn-off histaminergic activity. | Some evidence that the rate of histamine metabolism increases as estrogen levels increase;79 however, literature search did not locate studies that investigated a sleep/menstrual cycle/histamine connection. The question of whether changes in GABA due to progesterone changes could promote changes in histamine that lead to increased wakefulness remains. |
| Norepinephrine | Inactive during REM sleep. Related to loss of muscle tone. | In an animal study, progesterone was found to increase levels of norepinephrine.80 |
| Supplement | Action | Note |
| L-Theanine | May increase GABA levels by acting as an antagonist to glutamate receptors,111 and increasing conversion of glutamine into GABA.102 | Use cautiously with anti-hypertensive medications and hypotensive herbs and supplements (e.g. stinging nettle, CoQ10, and L-arginine). |
| L-Glutamine | Supplies glutamic acid/glutamate needed for GABA production. | Use cautiously if MSG hypersensitivity exists; contraindicated with history of cancer and/or use of anti-seizure medications. |
| 5-HTP | Serotonin enhances GABA activity and 5-HTP is an immediate precursor to serotonin (and ultimately melatonin); it freely crosses the blood-brain-barrier, unlike tryptophan, which requires a carrier (shared by other amino acids). | Do not take with antidepressants or other neurological drugs; consider after addressing other sleep hygiene issues; long-term use may deplete catecholamines, and it is important to include sufficient precursors for serotonin and dopamine.112 |
| Vitamin B6 | Co-factor for glutamate decarboxylase which synthesizes GABA from glutamate. | Consider both dietary intake and all supplements when making sure the tolerable upper limit (UL) of 100 mg/day is not exceeded. |
| Bifidobacterium | Produces GABA in the gut. | – |
| Lactobacillus | Produces GABA from glutamate. | “Lactobacillus brevis DPC6108 was the most efficient of the strains tested, converting up to 90% [corrected] of MSG to GABA.”113 |
| Factor | Goal | Rationale |
| Weight | Obtain or maintain a healthy weight. | Obesity is associated with irregular cycles, PCOS, and estrogen/progesterone imbalances. |
| Diet | Ensure adequate micronutrients (e.g. magnesium, vitamin B6), high fiber, and low-glycemic load. | Support production of neurotransmitters involved in sleep; support metabolism of sex hormones; avoid blood sugar spikes and dysregulation that can negatively affect sleep. |
| Assimilation | Optimize digestion and support gut diversity. | Support GABA-synthesizing bacteria; absorption of nutrients needed for neurotransmitter synthesis, methylation, etc. |
| Detoxification/biotransformation | Reduce exposure to endocrine disruptors. | Reduce estrogen/progesterone imbalances. |
| Stress resilience | Decrease perceived stress and increase stress resilience. | Psychological stress impairs the function of GABA,114 which could further exacerbate the effect of progesterone on GABA during the luteal phase; reduce cortisol level elevations that are associated with disrupted sleep; avoid depletions of magnesium and B vitamins associated with chronic stress. |
| Exercise | Develop a consistent exercise routine.Note: high intensity exercise in the evening may be counterproductive. | Support optimal circadian rhythms, balance sex hormone levels,115 support blood sugar regulation, increase stress resilience, and obtain/achieve healthy weight. |
| Sleep hygiene: Temperature | Offset the increase in CBT associated with the luteal phase. | Sleeping in a cool room or with a cooling blanket may reduce body temperature; warm baths before bedtime and the resulting drop can simulate the natural fall in temperature associated with efficient sleep onset. |
| Use of electronics | Reduce exposure to blue light several hours before bedtime by avoiding use of electronic devices, utilizing blue-light limiting applications, or wearing amber-colored glasses designed to block blue light. | Blue light suppresses melatonin production, which may be negatively impacted during the latter part of the luteal phase. |
| Education | Improve overall sleep hygiene. | Minimize any additional sleep disruptors. |
Hyperarousal and sleep reactivity in insomnia: current insights
Sleep disorders in women: clinical evidence and treatment strategies
Exploring sex and gender differences in sleep health: a Society for Women’s Health research report
Shining evolutionary light on human sleep and sleep disorders
The neuroprotective aspects of sleep
Neurophysiological effects of sleep deprivation in healthy adults, a pilot study
Slow-wave sleep and the risk of type 2 diabetes in humans
Premenstrual disorders
Premenstrual dysphoric disorder: epidemiology and treatment
Sleep regulation and sex hormones exposure in men and women across adulthood
Sleep, sleep disturbance, and fertility in women
Gender differences in sleep disorders
Unique aspects of sleep in women
Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones
The therapeutic potential of melatonin: a review of the science
New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation
Circadian and metabolic effects of light: implications in weight homeostasis and health
Circadian regulation of pineal gland rhythmicity
Role of the melatonin system in the control of sleep: therapeutic implications
The effectiveness of melatonin for promoting healthy sleep: a rapid evidence assessment of the literature
The dim light melatonin onset, melatonin assays and biological rhythm research in humans
Melatonin supplementation lowers oxidative stress and regulates adipokines in obese patients on a calorie-restricted diet
Photic regulation of melatonin in humans: ocular and neural signal transduction
Temperature as a universal resetting cue for mammalian circadian oscillators
Role of sleep-wake cycle on blood pressure circadian rhythms and hypertension
GABA mechanisms and sleep
Pharmacotherapy of insomnia
Main neuroendocrine features and therapy in primary sleep troubles
Hyperarousal and beyond: new insights to the pathophysiology of insomnia disorder through functional neuroimaging studies
Reduced brain GABA in primary insomnia: preliminary data from 4T proton magnetic resonance spectroscopy (1H-MRS)
Cortical GABA levels in primary insomnia
Interactions between evening and nocturnal cortisol secretion and sleep parameters in patients with severe chronic primary insomnia
Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications
Alterations in hypothalamus-pituitary-adrenal/thyroid axes and gonadotropin-releasing hormone in the patients with primary insomnia: a clinical research
Neuroendocrine regulation and metabolism of glucose and lipids in primary chronic insomnia: a prospective case-control study
Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders
Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis
Epigenetics: a new bridge between nutrition and health
The molecular and cellular effect of homocysteine metabolism imbalance on human health
Serotonin and the sleep/wake cycle: special emphasis on microdialysis studies
The neurotransmitters of sleep
Heterogeneity in HPA axis dysregulation and serotonergic vulnerability to depression
Correlation between cortisol level and serotonin uptake in patients with chronic stress and depression
Faulty cortisol/serotonin interplay. Psychopathological and biological characterisation of a new, hypothetical depression subtype (SeCA depression).
The effect of diet components on the level of cortisol
Integrative therapies in anxiety treatment with special emphasis on the gut microbiome
Sleep restriction for 1 week reduces insulin sensitivity in healthy men
An integrative review of sleep for nutrition professionals
The normal menstrual cycle and the control of ovulation
Menstrual cycle
Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer
Hormones in pregnancy
Progesterone prevents sleep disturbances and modulates GH, TSH, and melatonin secretion in postmenopausal women
Menstrual physiology: implications for endometrial pathology and beyond
The endocrine and paracrine control of menstruation
Endocrine regulation of menstruation
Sleep and women’s health
Actigraphy-defined measures of sleep and movement across the menstrual cycle in midlife menstruating women: study of Women’s Health Across the Nation Sleep Study
Menstrual cycle-related variation in physiological sleep in women in the early menopausal transition
Form and function of sleep spindles across the lifespan
Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women
Thalamocortical oscillations in the sleeping and aroused brain
Sleep, hormones, and circadian rhythms throughout the menstrual cycle in healthy women and women with premenstrual dysphoric disorder
Detection of ovulation, a review of currently available methods
Low plasma gamma-aminobutyric acid levels during the late luteal phase of women with premenstrual dysphoric disorder
Patients with premenstrual syndrome have a different sensitivity to a neuroactive steroid during the menstrual cycle compared to control subjects
Sedative and hypnotic effects of oral administration of micronized progesterone may be mediated through its metabolites
Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors
Review article: health benefits of some physiologically active ingredients and their suitability as yoghurt fortifiers
Selective serotonin reuptake inhibitors for premenstrual syndrome
Selective serotonin reuptake inhibitors for premenstrual syndrome
Treatment of premenstrual dysphoric disorder: therapeutic challenges
Stress-induced increases in progesterone and cortisol in naturally cycling women
Influence of menstrual cycle on platelet serotonin uptake site and serotonin2A receptor binding
The effects of estrogen and progesterone on blood glutamate levels: evidence from changes of blood glutamate levels during the menstrual cycle in women
Plasma concentration of acetylcholine in young women
Progesterone modulates a neuronal nicotinic acetylcholine receptor
Histamine in the regulation of wakefulness
Histamine metabolism and female sex hormones in women
Effect of progesterone on norepinephrine release from mouse adrenergic terminals in vitro
Objective sleep interruption and reproductive hormone dynamics in the menstrual cycle
Sleep quality and the sleep electroencephalogram in women with severe premenstrual syndrome
Effects of feet warming using bed socks on sleep quality and thermoregulatory responses in a cool environment
More than a marker: interaction between the circadian regulation of temperature and sleep, age-related changes, and treatment possibilities
Do sleeping disorders impair sexual function in married Iranian women of reproductive age? Results from a cross-sectional study
Adrenal responses to stress
Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity
The impact of sleep disorders on glucose metabolism: endocrine and molecular mechanisms
Sleep disturbances and insulin resistance
Polycystic ovary syndrome and the risk of obstructive sleep apnea: a meta-analysis and review of the literature
Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan
Polycystic ovary syndrome
Anxiety, depression, and quality of life in women with polycystic ovarian syndrome
Nonsteroidal anti-inflammatory drugs alter body temperature and suppress melatonin in humans
A possible change process of inflammatory cytokines in the prolonged chronic stress and its ultimate implications for health
Amber lenses to block blue light and improve sleep: a randomized trial
Bigger, brighter, bluer-better? Current light-emitting devices – adverse sleep properties and preventative strategies
[Prevention and treatment of sleep disorders through regulation of sleeping habits.] [In French]
Local body cooling to improve sleep quality and thermal comfort in a hot environment
Thermoregulation as a sleep signalling system
Night-time sleep EEG changes following body heating in a warm bath
Reduced stress and improved sleep quality caused by green tea are associated with a reduced caffeine content
Anti-stress, behavioural and magnetoencephalography effects of an l-theanine-based nutrient drink: a randomised, double-blind, placebo-controlled, crossover trial
5-Hydroxytryptophan: a clinically-effective serotonin precursor
Sleep-promoting effects of the GABA/5-HTP mixture in vertebrate models
Two combined amino acids promote sleep activity in caffeine-induced sleepless model systems
Vitamin B-B
The neuro-endocrinological role of microbial glutamate and GABA signaling
Microbiota and neurologic diseases: potential effects of probiotics
The probiotic characteristics and GABA production of Lactobacillus plantarum K154 isolated from kimchi
Involvement of GABA(A) receptors in the neuroprotective effect of theanine on focal cerebral ischemia in mice
5-HTP efficacy and contraindications
γ-Aminobutyric acid production by culturable bacteria from the human intestine
Effect of acute psychological stress on prefrontal GABA concentration determined by proton magnetic resonance spectroscopy
Effect of physical activity on sex hormones in women: a systematic review and meta-analysis of randomized controlled trials