Table 1
| Constituent | Specification (from manufacturer’s CofA) | Analysis (of test batch from manufacturer’s CofA) |
|---|---|---|
| Total curcuminoids (HPLC) | 18–22% | 18.3% |
| Water | <7% | 1.4% |
| Heavy metals (total USP) | <40 ppm | Complies |
| Residual solvents: ethanol | <5000 ppm | 910 ppm |
Table 2
| Constituent | Product specification (from manufacturer’s CofA) | Product label claims (of test batch from manufacturer’s CofA) |
|---|---|---|
| Turmeric root | 400 mg | |
| Turmeric root CO2 extract | 40% turmerones | 52.5 mg |
| Turmeric root alcohol extract | 95% curcuminoids | 102.5 mg |
| Black pepper extract | 95% piperine | 5 mg |
| Total curcuminoids | >95 mg | 95.5 mg |
| Total turmerones | >20 mg | 23.7 mg |
Table 3
| Sample analysis
|
||||
|---|---|---|---|---|
| LC Condition 1: | Curcumin | |||
| Column ID. & Dimensions: | ACQUITY UPLC BEH C8 1.7 μm, 50×2.1 mm (Waters) | Time (min)
|
% MPB
|
Flow (mL/min)
|
| Temperature (°C) | 55 | Initial | 10 | 1.0 |
| Mobile Phase A: | 0.1% Formic Acid in Water | 0.2 | 10 | 1.0 |
| Mobile Phase B: | 0.1% Formic Acid in Acetonitrile | 1.65 | 95 | 1.0 |
| Weak Needle Rinse: | 0.1% Formic Acid in Water | 1.9 | 95 | 1.0 |
| 1.95 | 10 | 1.0 | ||
| 2.00 | 10 | 1.0 | ||
| LC Condition 2: | Ranitidine | |||
|
|
||||
| Column ID. & Dimensions: | XSELECT HSS T3 2.5 μm, 30×2.1mm (Waters) | Time (min)
|
% MPB
|
Flow (mL/min)
|
| Temperature (°C) | 55 | Initial | 0 | 1.0 |
| Mobile Phase A: | 0.1% Formic Acid in Water | 0.15 | 0 | 1.0 |
| Mobile Phase B: | 0.1% Formic Acid in Acetonitrile | 0.8 | 95 | 1.0 |
| Weak Needle Rinse: | 0.1% Formic Acid in Water | 0.9 | 95 | 1.0 |
| 0.95 | 0 | 1.0 | ||
| 1 | 0 | 1.0 | ||
| LC Condition 3: | Talinolol & Warfarin | |||
|
|
||||
| Column ID. & Dimensions: | XSELECT HSS T3 2.5 μm, 30×2.1mm (Waters) | Time (min)
|
% MPB
|
Flow (mL/min)
|
| Temperature (°C) | 55 | Initial | 10 | 1.0 |
| Mobile Phase A: | 0.1% Formic Acid in Water | 0.15 | 10 | 1.0 |
| Mobile Phase B: | 0.1% Formic Acid in Acetonitrile | 0.8 | 95 | 1.0 |
| Weak Needle Rinse: | 0.1% Formic Acid in Water | 0.9 | 95 | 1.0 |
| 0.95 | 10 | 1.0 | ||
| 1 | 10 | 1.0 | ||
| LC Solution ID: | ||||
|
|
||||
| Mobile Phase A1: | 0.1% Formic Acid in Water | |||
| Mobile Phase A2: | NA | |||
| Mobile Phase B1: | 0.1% Formic Acid in Acetonitrile | |||
| Mobile Phase B2: | NA | |||
| Weak Needle Rinse: | 0.1% Formic Acid in Water | |||
| Strong Needle Rinse: | 25:25:25:25 Water:Acetonitrile:Methanol:Isopropanol | |||
| Seal Wash: | 0.1% Formic Acid in 95:5 Water:Acetonitrile | |||
| MS Conditions | ||||
|
|
||||
| Compounds | API5500 with Waters Acquity UPLC | |||
| Ionization Method: | Electrospray | |||
| Positive/Negative Ion: | Positive | |||
| Resolution: | Unit | |||
| Source Temperature (°C): | 600 | |||
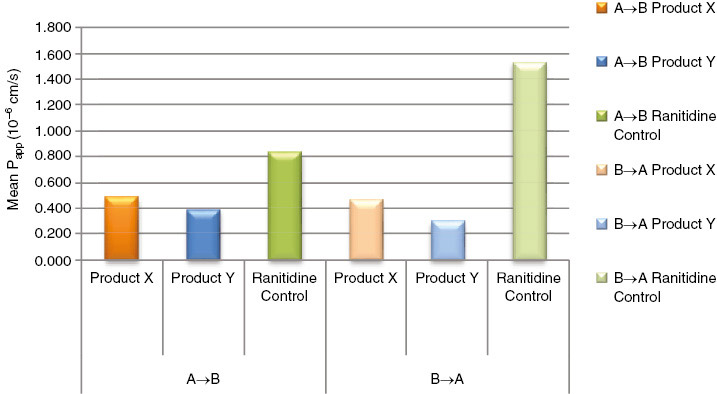
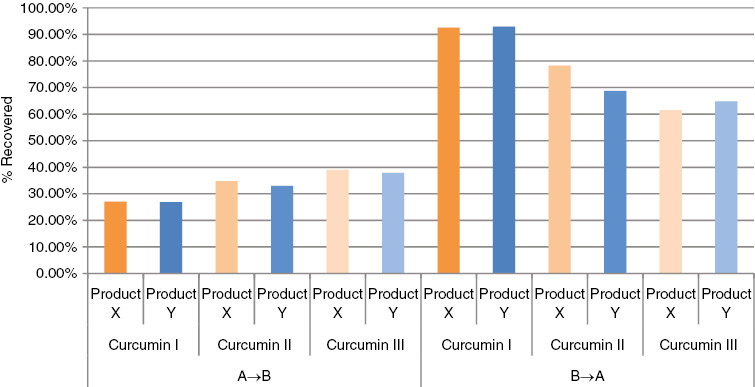
| Net efflux ratio
|
||
|---|---|---|
| Mean (B−A)/(A−B) | SEM | |
| Product Y curcumin I | 0.78 | 0.42 |
| Warfarin control | 0.91 | 0.09 |
| Product X curcumin I | 0.95 | 0.58 |
| Ranitidine control | 1.84 | 0.36 |
| Talinolol control | 7.83 | 0.51 |
| Talinolol+25 μM verapamil | 1.5 | 0.11 |
Bioavailability and activity of phytosome complexes from botanical polyphenols: the silymarin, curcumin, green tea, and grape seed extracts
The essential medicinal chemistry of curcumin
Dose escalation of a curcuminoid formulation
Recent advances and future prospects of phyto-phospholipid complexation technique for improving pharmacokinetic profile of plant actives
Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers
The role of turmerones on curcumin transportation and P-glycoprotein activities in intestinal Caco-2 cells
Phytosomes: the new technology for enhancement of bioavailability of botanicals and nutraceuticals
Herbosomes: herbo-phospholipid complex: an approach for absorption enhancement
Curcumin-phospholipid complex: preparation, therapeutic evaluation and pharmacokinetic study in rats
Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine
The Holy Grail of curcumin and its efficacy in various diseases: is bioavailability truly a big concern?
Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells
Efficacy and safety of Meriva, a curcumin–phosphatidylcholine complex, during extended administration in osteoarthritis patients
Potential role of curcumin phytosome (Meriva) in controlling the evolution of diabetic microangiopathy: a pilot study
Study of the intestinal absorption characteristics of curcumin in vivo and in vitro
Co-delivery of rapamycin- and piperine-loaded polymeric nanoparticles for breast cancer treatment